Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
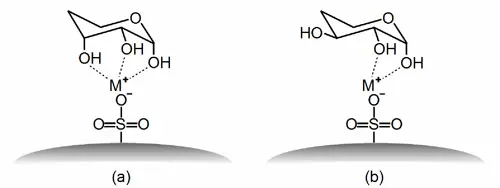
Ligand Exchange Chromatography
Ligand exchange chromatography is a specialised form of chromatography based on the interaction between a metal ion bound to the stationary phase and the analytes that can complex with this metal ion. This technique is mainly used to separate and analyse enantiomers and to separate molecules that are able to form stable complexes with the metal ion, such as certain amino acids, peptides, nucleotides and carbohydrates.
A significant advantage of ligand exchange chromatography is its high enantioselectivity, which makes it particularly valuable for chiral separation, a critical requirement in the pharmaceutical industry. With the ability to effectively separate optical isomers, this method supports drug development and quality control by ensuring that the therapeutically effective enantiomer is separated from potentially harmful isomers. In addition, ligand exchange chromatography offers excellent selectivity and sensitivity for the separation and analysis of biomolecules and metal ion-complexing compounds, making it a valuable tool in biochemical research, environmental analysis and food chemistry.
Write us a message and we will get back to you as soon as possible.