- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Affinity Chromatography
Affinity chromatography is a bioseparation technique that is used for the purification and analysis of proteins, nucleic acids or hormones, among other things. We have compiled a selection of available affinity chromatography columns from market leaders for you. Below you will find information on the base material, ligands and applications for the individual brand columns from Tosoh Bioscience, Shodex, Merck, Separation Methods Technologies (SMT) and Agilent. If you need further details or support, just contact us!
Technical Data
Basics of affinity chromatography
In affinity chromatography, the separation or purification of a substance is based on a highly specific biochemical interaction of the analyte with certain binding partners that are bound to the stationary phase (similar to antigen-antibody or enzyme-inhibitor interaction). Only one sample component in the existing substance mixture is retained by the stationary phase, while the other molecules can be easily rinsed off the column. At the end, the desired sample remains on the column bed, which can then be eluted by displacement, by changing the pH value or by changing the salt concentration.
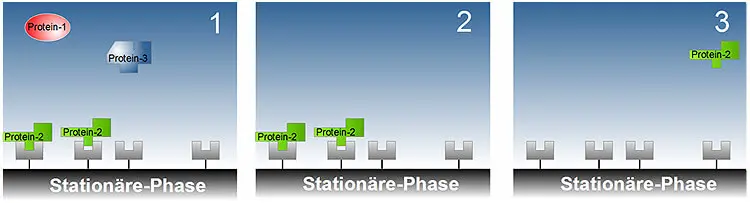
Figures: Schematic representation of the separation of three proteins using affinity chromatography: Figure 1: Only protein-2 can bind to the ligands of the stationary phase. Proteins-1 and -3 can be removed by washing; Figure 2: After elution of proteins-1 and -3, protein-2 alone remains on the column bed; Figure 3: Protein-2 is eluted under suitable conditions.
How do I choose the right affinity column?
As already described in the basics, the functionalisation of the column must exactly match the target analyte, as a reversible bond between the stationary phase and the target analyte is desired. Therefore, the modification of the affinity column is by far the most important criterion when selecting the column. As the target analytes are initially bound by the ligand under the starting conditions and all impurities are washed from the column, the columns are generally shorter compared to other types of chromtography. Other parameters play a more subordinate role.
Many recombinant proteins have a polyhistidine tag or a gluthathione S-transferase (GST) fusion tag.
The histidine tags bind to di- or trivalent metal ions. This type of affinity chromatography is known as Immobilised Metal Affinity Chromatography (IMAC). The metal commonly used for this is nickel, but copper, zinc and cobalt are also used. Imidazole is typically used as a competitive ligand for elution.
For GST fusion proteins, a tripeptide (Glu-Cys-Gly) can be used as a ligand for affinity chromatography. Gluthathione is used in the mobile phase to elute the bound protein.
Protein A is a frequently used ligand in affinity chromatography. Protein A binds to many immunoglobulins in many species. This is an easy way to purify antibodies from a matrix. For the elution of antibodies from a Protein A column, a mobile phase with a low pH and a high concentration of an amino acid such as glycine or arginine is normally used.
Protein G has a higher binding affinity compared to Protein A. Protein G binds optimally at acidic pH values, which results in elution under more acidic conditions (pH < 3). This high binding affinity can also result in IgG carry over, which is why Protein G should be selected specifically for the antibody.
Manufacturers and columns for affinity chromatography
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.
You will find:
Write us a message and we will get back to you as soon as possible.







