- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Hydrophobic Interaction Chromatography (HIC)
Hydrophobic interaction chromatography (HIC) is a special method used for the separation of biomolecules by utilising their hydrophobic properties. This method plays a central role in the purification and analysis of proteins, peptides and other macromolecules, especially when the preservation of the native structure and function of the molecules is important.
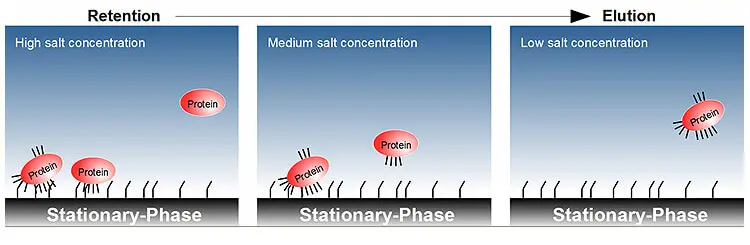
The separation is based on the interaction of hydrophobic regions of the proteins with a weakly hydrophobic stationary phase. The analytes are therefore separated according to their degree of surface polarity. Proteins with high surface hydrophobicity are more strongly retained by the stationary phase than those whose hydrophobic regions are found more in the interior of the protein. These interactions between protein and stationary phase are also greatly increased by the addition of salts to a buffered mobile phase, as the solubility of the proteins is reduced by the presence of the salts and thus the hydrophobic interactions of the protein with the stationary phase increase. The proteins are eluted from the column by a descending salt gradient. The mobile phase is purely aqueous and free of organic solvents, as these can lead to denaturation of the proteins to be analysed.
Here you will find an overview of manufacturers and HILC-HPLC columns. We will be happy to assist you in selecting the right column for your analytical problem!
Technical Data
How do I choose the right HIC pillar?
When choosing the right HIC column, it is usually a question of the base material, porosity and modification.
Polymers such as polymethacrylate are often used as the base material, but silica materials are also available. As organic solvents are not usually used in HIC, polymers are preferred due to their higher pH stability. However, if organic solvents are used, a silica column can also be selected as the material does not swell.
For porosity, a choice can be made between porous and non-porous materials. As HIC is mainly used for proteins, large pores are usually used. A porous material has a larger surface area and therefore a higher binding capacity. However, the retention times (or transit times) are somewhat longer as a result. Non-porous materials have a significantly smaller surface area and therefore a lower binding capacity. As a result, separation is quite fast.
- slow separation and high binding capacity → porous (e.g. preparative separation, lots of matrix)
- Fast separation and low binding capacity → non-porous (e.g. fast elution required, only analytical quantities)
The HIC phases have a slightly hydrophobic character. Butyl or phenyl ligands are usually used for this purpose, but other modifications such as C2, C3 or C6 are also available. The most commonly used ligand is the C4 (butyl) group. Phenyl can be used as an alternative with a less hydrophobic character. As a rough guide:
- less hydrophobic analytes → ligand with high hydrophobicity
- highly hydrophobic analytes → ligands with lower hydrophobicity
Manufacturer and columns of hydrophobic interaction chromatography
Shodex™
| Name | Functional Group | Particle Size | Pore Size | Column Size |
| HIC PH-814 | Phenyl | 10 µm | 2000 Å | 8.0 x 75 mm |
Base material: Polyhydroxymethacrylate
Tosoh Bioscience™
| Name | Functional Group | Particle Size | Pore Size | Column Size |
| TSKgel Ether 5-PW | Polyether | 10 µm | 1000 Å | 5.0 x 50 mm[1] |
| TSKgel Phenyl 5-PW | Phenyl | 10 µm | 1000 Å | 5.0 x 50 mm[1] |
| TSKgel BioAssist Phenyl | Phenyl | 10 µm | 1000 Å | 7.8 x 50 mm[4] |
| TSKgel Butyl-NPR | Butyl | 2.5 µm | Non-porous | 4.6 x 35 mm[2] |
Base material: polymethacrylates; [1] glass columns; [2] stainless steel columns; [3] particle size 13 µm; [4] PEEK column
Thermo Scientific™
| Name | Functional Group | Particle Size | Pore Size | Column Size |
| MAbPac HIC-10[1] | Proprietary polyamide | 5 µm | 1000 Å | 4.6 x 100 mm |
| MAbPac HIC-20[1] | Proprietary alkylamide | 5 µm | 1000 Å | 4.6 x 100 mm |
| MAbPac HIC-Butyl[2] | Butyl | 5 µm | Non-porous | 4.6 x 100 mm |
| ProPac HIC-10[1] | Proprietary alkylamide | 5 µm | 300 Å | 7.8 x 75 mm |
[ 1] Base material: Silica gel; [2] Base material: Hydrophilic polymer
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.
You will find:
Write us a message and we will get back to you as soon as possible.
